 |  |  | Causes of eutrophication |
In general, the nutrient elements limiting the primary production in freshwater is phosphorus (mainly phosphate) while that in the marine environments is nitrogen (mainly nitrate). In the marine environment, exceptions have been reported to this general rule. Thus the eastern Mediterranean Sea and many eutrophied estuaries are P rather than N limited, while the equatorial Pacific and extensive regions around Antarctica appear to be Fe-limited. What are the sources of nutrients to aquatic ecosystems? There is nutrient supply from
28% of the annual N fixation of the global biosphere is caused by nitrogen fertilizer production, which is energetically expensive and largely based on fossil fuel consumption. A three-fold increase in N utilization by agriculture in Western Europe and USA has been recorded between 1950 and 1970 on agricultural land that actually declines because increased efficiency or over-exploitation (Figure 2). Animal waste from intensive husbandry is of particular significance for nutrient point sources: considerable amounts are directly supplied to freshwater and fields. There are also significant losses by NH3 emission and denitrification on fields, rivers or shallow estuaries, connecting the agricultural lands directly to the atmosphere. Agricultural run-off has given rise to significant eutrophication in most estuaries, but also in entire coastal seas such as the southern North Sea, Baltic Sea, Kattegat, northern Adriatic Sea, Chesapeake Bay and Seto Inland Sea in Japan.
Aquaculture techniques are applied to restricted areas such as straights, fjords and rias where it, in the worst case, may induce anoxia (if hydrodynamic energy supply is insufficient). C and N supply is normally regulated by environmental control, but can be detrimental in some areas. It has been reported that accumulation rates of fat- and N-rich food and faeces at the bottom below caged fish production sites could make up to 10 cm per month! As much as 30 and 40% of the annual discharge of P and N, respectively, is caused by aquaculture in some fjords. Unless an entire region is used for aquaculture, it has `local' consequences for both plankton and benthos. However, large factories of the food industry can discharge significant amount of dissolved and particulate organic matter as well as nutrients into the recipient that can represent important point sources.
Close to cities and dense populated areas sewage is of utmost significance, but compared to run-off from agricultural drainage basins, point sources are of less significance; see alsoagricultural runoff section). The emphasis given to sewage treatment in many regions is in contrast to the inefficient and at time completely lacking emphasis on the largest nutrient source for aquatic recipients, i.e. the agriculture. Sewage discharge has `local' consequences, although local could mean entire estuaries, river mouths and fjords. The frequent removal of P by sewage plants and the decline of utilization of P fertilizers (in contradiction to N, Figure 3) results often in an excess supply of N. Consequently, marine recipients are forced into P rather than N limitation. Far more emphasis has been given to sewage treatment than manipulations of effluents from agriculture, and this can be partly explained by the relative simplicity of removing nutrients from point sources.
River-run off has changed significantly over the last 200 years in many region of the world. Large-scale manipulation of lower reaches of river has resulted in greater river speeds. The residence times of water in the Rhine river water shed was far greater before 1850 when it was so shallow and meandering south of the town of Strasbourg that one could walk through it even during flood times! Extensive wetlands have been removed in favour of shipping and straightforward navigation. Both agriculture and logging result in increased erosion as trees, bushes and vegetation is reduced. Removal of wetlands for agricultural purposes results in decreasing self-purification as denitrification decreases. As a consequence, we experience an increased contribution of particulate matter and nutrients to estuaries. Due to various practices, N supply to marine waters does still increase in Western Europe, while P supply decreases (Figure 3). As a consequence P limitation in eutrophicated coastal regions increases.
Lately, more focus has been given to the role of the atmosphere affecting the availability of nutrients in aquatic ecosystems (see atmospheric deposition section). Generally, nutrient supply from the atmosphere is in the form of N. Only in case dust is deposited particulate P is deposited atmospherically. Atmospheric deposition of nutrients is partly due to fossil energy application, leading to the emission of NOx gasses to the atmosphere. Here these gasses are transformed to nutrients such as NO3 that can be deposited as wet or dry deposition. However, much of the atmospheric nutrient deposition comes from the emission of NH4 by intensive husbandry and meat production. The annual atmospheric supply of N to open Skagerrak is more than 30% of N the budget! It is relatively lower in the winter months when run-off from land is prominent, but can be the dominating N source during summer. Atmospheric N deposition is closely reflected to source regions, the movements of air masses and the low-pressure pathways. Atmospheric deposition of N gives rise to a wide spread eutrophication of freshwater and terrestrial environments as well as the sea. Eutrophication due to use of fossil energy depends on emission and precipitation pattern.
During nutrient limiting conditions N fixation can be an important source of N in fresh water and brackish water bodies such as the Baltic Sea. The significance of N fixation is still a matter of discussion in marine environments. However, the global N2 cycle has changed greatly due to the production of fertilizers as human N2 fixation is now in the same order of magnitude than the calculated global fixation.
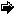 |  |  | Causes of eutrophication |